LG Chem Life Sciences Company’s Gout Drug Development History Interview Vol. 2 – ‘LG Chem develops a new gout treatment, Tigulixostat, based on our global clinical trial competency!’
2023. 12. 08
LG Chem Life Sciences Company’s Gout Drug Development History Interview Vol. 2 – ‘LG Chem develops a new gout treatment, Tigulixostat, based on our global clinical trial competency!’
2023. 12. 08
Gout is a disease that causes pain just by a brush of wind. The global prevalence rate of gout is under 6~8%, however, the number of patients is constantly rising across all age groups. Although gout is more common in men than women, women need also be aware of this disease after reaching the menopause. Gout can attack not only patients with obesity, but also those with thin or muscular bodies.
Meanwhile, LG Chem’s in-house developed new gout drug, Tigulixostat, has entered phase 3 of the clinical trial. In the previous episode, we heard from Professional Jang Young-hwan, who is in charge of the clinical trial. Today, we invite CMC* Project Leader and XO* Project manager to the 2nd interview session of our gout drug development project.
*CMC: Abbreviation for Chemistry, Manufacturing, and Control. It refers to the process development and quality management of pharmaceuticals.
*XO: Xanthine Oxidase, catabolic enzyme involved in purine metabolism which produces uric acid
Hello! Please introduce yourself.

•Kim Ri-seon, Professional: Hello, I’m Professional Kim Ri-seon from the formulation technology team. I joined LG Chem Life Sciences Company in 2008, and since then, I’ve been in charge of the formulation of our drugs. I’m also working as a CMC PL for the XO project since 2019.
•Jeon Ji-seon, Specialist: Hello, I’m Specialist Jeon Ji-seon, responsible for the XO development of the overall drug development.
What is your role in the team?
•Kim Ri-seon, Professional: After joining the LG chem, I only studied the drug formulation, but based on my experience in formulation research in 2019, I started to carry out the work of CMC PL together. I participated in the development of Tigulixostat (here below, TGX) through formulation study, Scale up production, and clinical drug manufacture. In addition, I’m also in charge of reviewing the clinical drug labels, writing documents for authorization, managing the schedules and expenses of the CMC part, and technology transfer for in preparation for commercialization.
•Jeon Ji-seon, Specialist: I manage all activities regarding TGX, including the timeline and budget. And end of last year we signed a license agreement with China and so I am also responsible for alliance management which includes communication with our partner for efficient and smooth development and commercialization of TGX in China. TGX has now entered phase 3 of clinical trial, therefore, we are getting closer to commercialization. I’m also closely collaborating with commercial teams to prepare for the commercialization in advance.
The new gout drug development must have called for participation of many departments. Could you tell us about the cooperation?

•Jeon Ji-seon, Specialist: In order to develop a new drug, we must cooperate with nearly all departments within the company. To start with, we communicate with the research organizations that manage clinical trial/research/authorization. Also, new drug development is subject to a great amount of investment. Therefore, we work closely with the investment management team. Outside the company, we carry out pre-marketing activities to inform doctors and future patients about the Unmet needs of gout and LG Chem Life Sciences as a Company. While doing this, we join hands with Medical Affairs and the marketing team. Furthermore, gout drug requires partnership with other companies, as well. Recently, LG Chem signed a license contract with Chinese pharmaceutical company INNOVENT to seek business opportunity of new gout drug in the Chinese market. We’re carrying out a steady communication with INNOVENT so that TGX can be smoothly developed and launched in China.
You could easily understand the size of TGX project by learning how many people and size of the budget. Currently, there are more than 100 personnel working for phase 3 of the clinical trial, and if we count the number of people who participated in the initial material development stage, it goes beyond our calculations. It’s a massive project where we input a budget of approximately 300 million USD.
There are existing gout drugs that were developed before. What distinguishes LG Chem’s TGX from them?
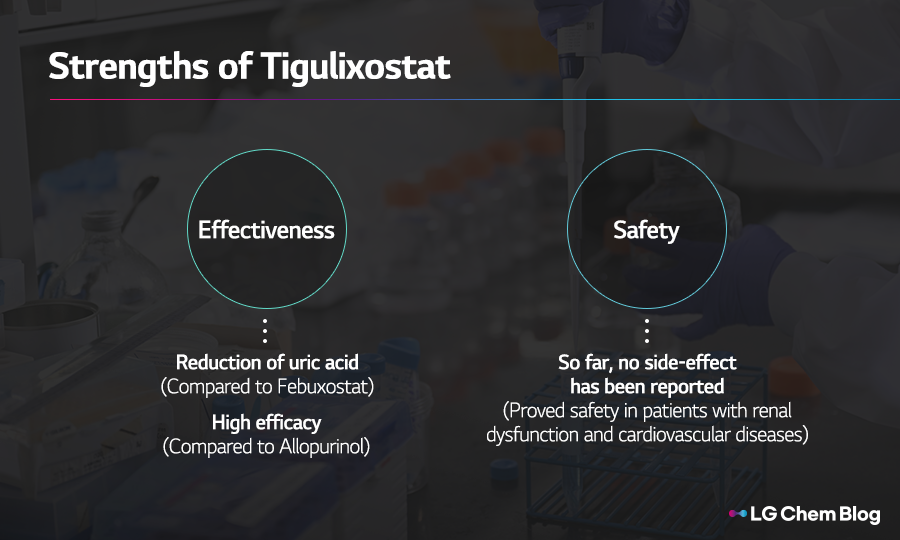
•Kim Ri-seon, Professional: Our new gout drug, TGX, has proven high effectiveness and safety compared to its competitors.
As for the effectiveness, the phase 2 clinical trial results have proven that TGX can reduce uric acid more effectively than the existing gout drug, Febuxostat. Also, the comparative study between TGX and its another competitor, Allopurinol, will be conducted to confirm TGX’s efficacy .
Next, in terms of safety, TGX has not reported any adverse events unlike Febuxostat which poses risks of cardiovascular diseases.
What’s left is testing our new drug in larger and more diverse group of gout patients in the last clinical phase and evaluate the final safety and effectiveness of TGX. However, we’re expecting that TGX will not have special issues concerning its safety, and yet prove higher effectiveness compared to existing drugs.
What are possible challenges in passing the phase 3 clinical trial? If you succeed in developing the new gout drug despite all difficulties, what prospects do you have?

•Kim Ri-seon, Professional: The very-last hurdle of a new drug development is completing phase 3 of clinical trial. Many candidate drugs fail to vault over this stage. From a manufacturer’s perspective, it’s important that we manage the Contract Manufacturing Organization (CMO) in order to supply the clinical drug without any issue and at the right time. Additionally, from the clinical perspective, there’s a challenge of collecting about 3,000 patients within limited time and obtaining clinical approval from each country.
With the success of TGX development, LG Chem will see its 3rd self-developed drug in the market. Considering the XO project, TGX is more meaningful that it’s our first case of conducting multi-regional clinical trials. In other words, it’s the cornerstone of LG Chem’s entry to global market. With XO project as the strong foundation, we expect to gain speed in developing other new candidate drugs.
•Jeon Ji-seon, Specialist: Gout treatment usually takes a palliative approach to ease the symptoms. However, TGX is focused on treating the fundamental cause of the disease. With active pre-marketing and academic activities, we’re informing more people of the importance of fundamental treatment of gout. When the new gout drug is finally commercialized, we’re hoping that the market perception of the need for fundamental gout treatment will be higher, and patients will welcome the release of our new drug.
What would you boast about LG Chem Life Sciences Company?

•Kim Ri-seon, Professional: LG Chem Life Sciences Company is more focused on R&D of new drugs than generics. Unlike other pharmaceutical companies, we’re equipped with professional teams that carry out R&D of new drugs. Our experts already hold two times of experience in developing new drugs, which gives us an optimal condition for studying new drugs. In addition, LG Chem is investing up to 26% of their sales in the R&D of Life Sciences Company, which means we’re receiving a full support from the company. Our current sales may not be the highest, but it’s constantly increasing. As the scale of R&D investment keeps rising, we can expect to see more from this company in the future.
What are your future goals to pursue in LG Chem?

•Kim Ri-seon, Professional: In the short term, I want to successfully release TGX in the United States. In the long term, I want to use my interest in rare drugs to develop treatments for patients suffering from rare diseases. Based on my experience of developing TGX, I want to do my best in my job to create the 2nd and 3rd TGX.
•Jeon Ji-seon, Specialist: My goal is to tune the communication among members participating in the TGX project until it is successfully commercialized. In particular, I’d be thrilled to see TGX commercialization with INNOVENT in China.
That’s the end for the interview with LG Chem Life Sciences gout drug development project team, who are pouring their efforts into developing TGX with high safety and effectiveness. According to the research done by Coherent Market Insights, the global gout treatment market is expected to expand from $2.6 billion in 2019 to $4.3 billion in 2027, due to aging and the rise in obesity. In the expanding gout drug market, we can expect that TGX will be the foothold for LG Chem to reinforce our competency regarding clinical trial, authorization, production, and sales. Until TGX vaults over phase 3 of clinical trial and gains approval to be of actual help for gout patients, LG Chem will do our best for a healthier life of our patients.

There are no comments yet! Be the first to let us know your thoughts!